To better understand ocean acidification and the effects on shellfish SCCOOS, along with AOOS, NaNOOS and CeNCOOS, partnered with the shellfish industry to test state-of-the-art carbon system instruments, such as the Burkolator, at hatcheries and shellfish growing sites, as well as transition more affordable sensors (e.g., ACDC and SeapHOx) to operations. The full asset list is available on the IOOS Partners Across Coasts Ocean Acidifcation (IPACOA) data portal.
Locations
The ACDC was operated alongside two SeapHOx units measuring pH, temperature and salinity and the Burkolator. The calibrated SeapHOx pH data were then combined with the salinity-derived alkalinity to generate a continuous pCO2 value for comparison with the ACDC system. The SeapHOx units, functioning in real time, provided more accurate pH data in comparison to the ACDC.
The Burkolator was measuring eight OA variables at the Carlsbad Aquafarm in the Agua Hedionda Lagoon from January 2017 to June 2019. The generation 1 ACDC was transitioned to Catalina Sea Ranch NOMAD buoy in March 2019 and the Burkolator is now being transitioned to University of California Davis. The SeapHOx unit contines to monitor pH data in real time at Carlsbad Aquafarm.
Technologies
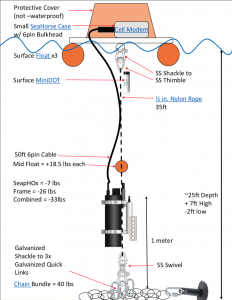
SeapHOx
- Alkalinity (derived from Salinity)
- O2
- pCO2 (derived from pH and salinity-derived alkalinity)
- pH
- Pressure
- Salinity
- Temperature
Real-time data from a SeapHOx sensor deployed in Agua Hedionda Lagoon near the Carlsbad Aquafarm. The SeapHOx measures pH, dissolved oxygen, temperature, conductivity, and pressure, and data are combined with an average total alkalinity from bottle samples to derive the saturation state of aragonite.
Applications
1. Ecosystems and Fisheries
While the impacts of ocean acidification are vast, organisms that rely on the carbonate ion to build their shells such as clams, oysters, urchins, and even coral reefs are at great risk. The decreasing concentrations of the carbonate ion can make maintaining shells more difficult and increasing levels of acidity prove to be corrosive and harmful to such organisms. Another organism that faces the impact of ocean acidification is phytoplankton. Even though these organisms are microscopic, their response to changing levels in pH can have a drastic bottom-up effect on marine ecosystems. Some species of phytoplankton will thrive and others will die out due to competition and available resources. This imbalance of phytoplankton species will influence what higher trophic levels with members such as fish, will be present. This trend will continue up the food web, eventually affecting local fisheries and aquafarms.
2. Climate Variability and Change
Ocean acidification has the potential to fundamentally change the ocean, its habitats, food webs, and marine life. The NOAA Ocean Acidification Program and IOOS west coast Regional Associations are monitoring the changes in the ocean chemistry, researching potential effects on organisms and ecosystems, and understanding the socio-economic impacts of those changes.
Data Access
ERDDAP Burkolator
THREDDS Burkolator
Burkolator Dashboard
ERDDAP SeapHOx Sensor
ACDC Dashboard
Principal Investigator
Todd Martz, UCSD - trmartz @ucsd.edu
Publications
Bresnahan, P.J., Wirth, T., Martz, T., Shipley, K., Rowley, V., Anderson, C., Grimm, T. 2020. Equipping smart coasts with marine water quality IoT sensors. Results in Engineering, 5. https://doi.org/10.1016/j.rineng.2019.100087
Martz, T., K.L. Daly, R.H. Byrne, J.H. Stillman, and D. Turk. 2015. Technology for ocean acidification research: Needs and availability. Oceanography 28(2):40–47, https://doi.org/10.5670/oceanog.2015.30.
Bresnahan, P.J. Martz T.R., Takeshita Y., Johnson K.S., LaShomb M. 2014. Best practices for autonomous measurement of seawater pH with the Honeywell Durafet, Methods in Oceanography, 9 44-60. https://doi.org/10.
Martz, T., Send, U., Ohman, M. D., Takeshita, Y., Bresnahan, P., Kim, H. J., & Nam, S. 2014. Dynamic variability of biogeochemical ratios in the Southern California Current System. Geophysical Research Letters, 41(7), 2496-2501. https://doi.org/10.1002/2014GL059332
Frieder, C. A., Nam, S. H., Martz, T., & Levin, L. A. 2012. High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeosciences, 9(10), 3917-3930. https://doi.org/10.5194/bg-9-3917-2012
Hofmann, G. E., Smith, J. E., Johnson, K. S., Send, U., Levin, L. A., Micheli, F., ... & Martz, T. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PloS one, 6(12), e28983. https://doi.org/10.1371/journal.pone.0028983
Kroeker KJ, Micheli F, Gambi MC, Martz T. 2011. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proceedings of the National Academy of Sciences, 108, 14515–20. https://doi.org/10.1073/pnas.1107789108